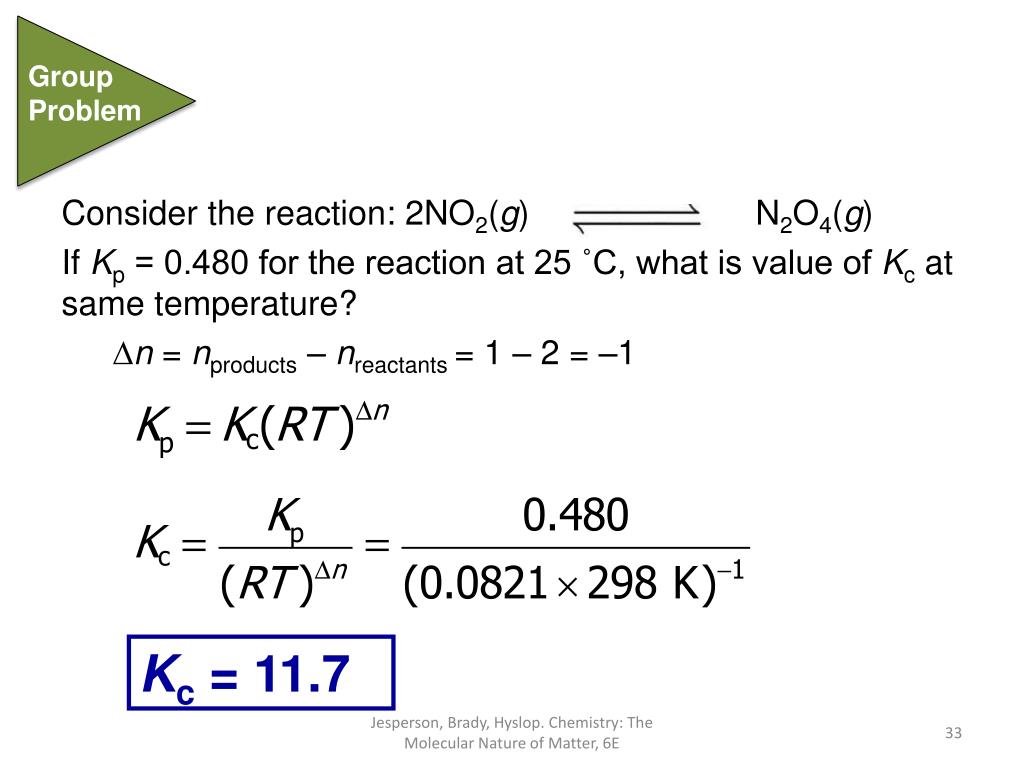
How To Find Kc And Kp In Chemistry . We saw in the exercise in example 6 in section 15.2 that. the relationship between kp and kc. this chemistry video tutorial on chemical equilibrium explains how to calculate kp from kc using a simple formula.chemical. calculating an equilibrium constant from equilibrium concentrations. Mol), t = kelvin temperature, and δn = the change in. relationship between k_p and k: For the equilibrium a a + b b c c + d. Kc = [right hand side]eq. The equilibrium constant (k) the law of mass action. K_p = k (rt)^ {δn} \nonumber. The relationship between kc and kp is shown below: how the gas equilibrium constants relate to reaction quotient (q) the process of finding the reaction quotient (q c) is the same as finding k c and k p, where. [ ] refers to the concentration of the species. kc is the equilibrium constant. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p.
from slidesharenow.blogspot.com
the relationship between kp and kc. The relationship between kc and kp is shown below: relationship between k_p and k: Although the values of kp and kc are generally different, it is possible to calculate one from the other. this chemistry video tutorial on chemical equilibrium explains how to calculate kp from kc using a simple formula.chemical. how the gas equilibrium constants relate to reaction quotient (q) the process of finding the reaction quotient (q c) is the same as finding k c and k p, where. calculating an equilibrium constant from equilibrium concentrations. We saw in the exercise in example 6 in section 15.2 that. R = universal gas constant (0.0821 l. K_p = k (rt)^ {δn} \nonumber.
What Is Kc In Chemistry slideshare
How To Find Kc And Kp In Chemistry We saw in the exercise in example 6 in section 15.2 that. kc is the equilibrium constant. R = universal gas constant (0.0821 l. Although the values of kp and kc are generally different, it is possible to calculate one from the other. K_p = k (rt)^ {δn} \nonumber. Mol), t = kelvin temperature, and δn = the change in. Kc = [right hand side]eq. For the equilibrium a a + b b c c + d. how the gas equilibrium constants relate to reaction quotient (q) the process of finding the reaction quotient (q c) is the same as finding k c and k p, where. relationship between k_p and k: the relationship between kp and kc. calculating an equilibrium constant from equilibrium concentrations. this chemistry video tutorial on chemical equilibrium explains how to calculate kp from kc using a simple formula.chemical. We saw in the exercise in example 6 in section 15.2 that. The equilibrium constant (k) the law of mass action. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p.
From www.wizeprep.com
Kc vs Kp Wize University Chemistry Textbook Wizeprep How To Find Kc And Kp In Chemistry kc is the equilibrium constant. We saw in the exercise in example 6 in section 15.2 that. Although the values of kp and kc are generally different, it is possible to calculate one from the other. relationship between k_p and k: This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Mol), t. How To Find Kc And Kp In Chemistry.
From www.bharatagritech.com
Learn How The Equilibrium Constants Kc And Kp Are Chemistry, 51 OFF How To Find Kc And Kp In Chemistry This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. The relationship between kc and kp is shown below: Although the values of kp and kc are generally different, it is possible to calculate one from the other. calculating an equilibrium constant from equilibrium concentrations. K_p = k (rt)^ {δn} \nonumber. [ ] refers. How To Find Kc And Kp In Chemistry.
From haipernews.com
How To Find Kc If Kp Is Given Haiper How To Find Kc And Kp In Chemistry kc is the equilibrium constant. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. R = universal gas constant (0.0821 l. Kc = [right hand side]eq. For the equilibrium a a + b b c c + d. Mol), t = kelvin temperature, and δn = the change in. [ ] refers to. How To Find Kc And Kp In Chemistry.
From www.vrogue.co
Kp And Kc Relationship Chemistry Steps vrogue.co How To Find Kc And Kp In Chemistry Although the values of kp and kc are generally different, it is possible to calculate one from the other. Kc = [right hand side]eq. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. the relationship between kp and kc. The equilibrium constant (k) the law of mass action. this chemistry video tutorial. How To Find Kc And Kp In Chemistry.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube How To Find Kc And Kp In Chemistry K_p = k (rt)^ {δn} \nonumber. kc is the equilibrium constant. For the equilibrium a a + b b c c + d. Kc = [right hand side]eq. The relationship between kc and kp is shown below: The equilibrium constant (k) the law of mass action. the relationship between kp and kc. R = universal gas constant (0.0821. How To Find Kc And Kp In Chemistry.
From haipernews.com
How To Calculate Kc And Qc Haiper How To Find Kc And Kp In Chemistry The relationship between kc and kp is shown below: This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. the relationship between kp and kc. Mol), t = kelvin temperature, and δn = the change in. kc is the equilibrium constant. K_p = k (rt)^ {δn} \nonumber. Although the values of kp and. How To Find Kc And Kp In Chemistry.
From www.youtube.com
How to Write Equilibrium Constant Expression Kc and Kp Expression How To Find Kc And Kp In Chemistry kc is the equilibrium constant. relationship between k_p and k: R = universal gas constant (0.0821 l. We saw in the exercise in example 6 in section 15.2 that. K_p = k (rt)^ {δn} \nonumber. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. this chemistry video tutorial on chemical equilibrium. How To Find Kc And Kp In Chemistry.
From lucabaldwin56.blogspot.com
Relationship Between Kp And Kc Relationship Between Kc, Kp, Kx and Kn How To Find Kc And Kp In Chemistry Kc = [right hand side]eq. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. [ ] refers to the concentration of the species. the relationship between kp and kc. K_p = k (rt)^ {δn} \nonumber. this chemistry video tutorial on chemical equilibrium explains how to calculate kp from kc using a simple. How To Find Kc And Kp In Chemistry.
From cloudshareinfo.blogspot.com
Kp Kc Rt N cloudshareinfo How To Find Kc And Kp In Chemistry This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. the relationship between kp and kc. Mol), t = kelvin temperature, and δn = the change in. [ ] refers to the concentration of the species. The relationship between kc and kp is shown below: We saw in the exercise in example 6 in. How To Find Kc And Kp In Chemistry.
From www.youtube.com
Week 6 15. Calculating Kc from Kp YouTube How To Find Kc And Kp In Chemistry the relationship between kp and kc. Mol), t = kelvin temperature, and δn = the change in. The relationship between kc and kp is shown below: Although the values of kp and kc are generally different, it is possible to calculate one from the other. how the gas equilibrium constants relate to reaction quotient (q) the process of. How To Find Kc And Kp In Chemistry.
From www.vrogue.co
Kp And Kc Relationship Chemistry Steps vrogue.co How To Find Kc And Kp In Chemistry [ ] refers to the concentration of the species. We saw in the exercise in example 6 in section 15.2 that. Kc = [right hand side]eq. calculating an equilibrium constant from equilibrium concentrations. R = universal gas constant (0.0821 l. The equilibrium constant (k) the law of mass action. how the gas equilibrium constants relate to reaction quotient. How To Find Kc And Kp In Chemistry.
From slidesharenow.blogspot.com
What Is Kc In Chemistry slideshare How To Find Kc And Kp In Chemistry kc is the equilibrium constant. Kc = [right hand side]eq. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Although the values of kp and kc are generally different, it is possible to calculate one from the other. calculating an equilibrium constant from equilibrium concentrations. K_p = k (rt)^ {δn} \nonumber. We. How To Find Kc And Kp In Chemistry.
From haipernews.com
How To Calculate Kc When Given Kp And Temperature Haiper How To Find Kc And Kp In Chemistry The equilibrium constant (k) the law of mass action. the relationship between kp and kc. Kc = [right hand side]eq. The relationship between kc and kp is shown below: R = universal gas constant (0.0821 l. For the equilibrium a a + b b c c + d. Although the values of kp and kc are generally different, it. How To Find Kc And Kp In Chemistry.
From www.chegg.com
Solved For a given reaction, how are Kc and Kp related? How To Find Kc And Kp In Chemistry kc is the equilibrium constant. the relationship between kp and kc. K_p = k (rt)^ {δn} \nonumber. Although the values of kp and kc are generally different, it is possible to calculate one from the other. The equilibrium constant (k) the law of mass action. relationship between k_p and k: We saw in the exercise in example. How To Find Kc And Kp In Chemistry.
From www.toppr.com
Calculate Kc and Kp for the reaction at 295 K, N2O4 (g) 2NO2(g) if the How To Find Kc And Kp In Chemistry The equilibrium constant (k) the law of mass action. Kc = [right hand side]eq. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. K_p = k (rt)^ {δn} \nonumber. For the equilibrium a a + b b c c + d. calculating an equilibrium constant from equilibrium concentrations. this chemistry video tutorial. How To Find Kc And Kp In Chemistry.
From lucabaldwin56.blogspot.com
Relationship Between Kp And Kc Relationship Between Kc, Kp, Kx and Kn How To Find Kc And Kp In Chemistry Although the values of kp and kc are generally different, it is possible to calculate one from the other. The relationship between kc and kp is shown below: For the equilibrium a a + b b c c + d. calculating an equilibrium constant from equilibrium concentrations. [ ] refers to the concentration of the species. kc is. How To Find Kc And Kp In Chemistry.
From chem.libretexts.org
Gas Equilibrium Constants Kc And Kp Chemistry LibreTexts How To Find Kc And Kp In Chemistry K_p = k (rt)^ {δn} \nonumber. The relationship between kc and kp is shown below: For the equilibrium a a + b b c c + d. R = universal gas constant (0.0821 l. relationship between k_p and k: kc is the equilibrium constant. how the gas equilibrium constants relate to reaction quotient (q) the process of. How To Find Kc And Kp In Chemistry.
From chemistryskills.com
What Is Kc In Chemistry? Chemistry Skills How To Find Kc And Kp In Chemistry calculating an equilibrium constant from equilibrium concentrations. this chemistry video tutorial on chemical equilibrium explains how to calculate kp from kc using a simple formula.chemical. The equilibrium constant (k) the law of mass action. [ ] refers to the concentration of the species. K_p = k (rt)^ {δn} \nonumber. kc is the equilibrium constant. relationship between. How To Find Kc And Kp In Chemistry.